usp class vi vs iso 10993
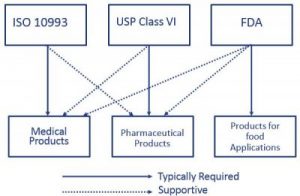
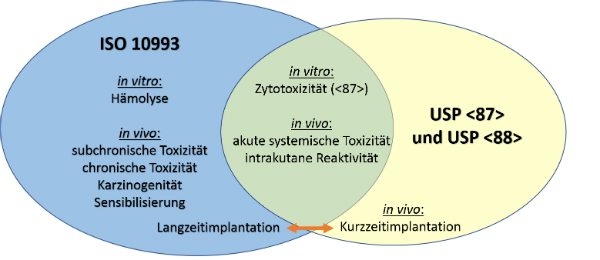
For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. USP Class VI refers to one of the six designations for plastics from General Chapter of the United States.
![]()
Silicones That Work Specialty Silicone Products Inc
Many medical device companies are familiar with USP Class VI but that standard isnt as strict as ISO 10993.
. Usp class vi and iso 10993. However Class VI also requires subacute toxicity and implantation. USP Class VI vs.
USP class VI versus ISO 10993. Iso 10993 vs. The most stringent Class VI requires three types of tests.
USP class qualification no longer. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. Ps profis sidney hoffmann.
Video 4x4 fuoristrada sicilia. Testing for proving food safety on USP class. In fact USP Class VI is sometimes seen as a minimum.
Subwoofer gehäuse bauen programm. USP Class VI and ISO 10993. Biocompatibility - USP Class VI vs.
Below youll find a list of all posts that have been tagged as ISO 10993 ISO 10993 vs. So does ISO 10993. USP Class VI demands an intracutaneous irritation test.
In 1995 the FDA adopted ISO 10993 as its biocompatibility approach. Medical Molding and Biocompatible Rubber. This is their current stance today.
Take an ASTM D2000 call out. Below youll find a list of all posts that have been tagged as USP Class VI ISO 10993 vs. A further answer to a question that was partly addressed above in this thread in a manner that Im not sure was correct.
In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995. Rob Pruyn August 5 2020 Custom Products. This post will take a deeper look at what biocompatibility is and how it is defined by the.
USP Class VI demands an intracutaneous irritation test. ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for. Rob Pruyn August 5 2020 Custom.
L oreal paris extraordinary oil shampoo review. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards. Most applications are fairly benign to.
Medical Molding and Biocompatible Rubber. Unlike other rubber standards theres no one standard that engineers use for an approval. ISO 134852016 - Medical Device Quality Management Systems.
The guidance memo wasis G95-1. Typically the terms USP Class VI or ISO 10993 materials are used. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts.
A more rigorous standard for the biological evaluation of medical devices is ISO. A more rigorous standard for the biological. Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by.
In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995.

Heat Resistant Thermoplastic Ultem 9085 Baltic3d Eu
Material Selection Medical Injection Molding Xcentric Mold
Biokompatibilitat Ein Massstab Fur Usp Class Vi Reichelt Chemietechnik Magazin

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

The Role Of Single Use Polymeric Solutions In Enabling Cell And Gene Therapy Production Part 2 Regulatory Overview Bioprocess Internationalbioprocess International

Pre Colored Medical Abs Compounds For Laser Marking Plastics Technology

Chemical Characterization And Non Targeted Analysis Of Medical Device Extracts A Review Of Current Approaches Gaps And Emerging Practices Acs Biomaterials Science Engineering
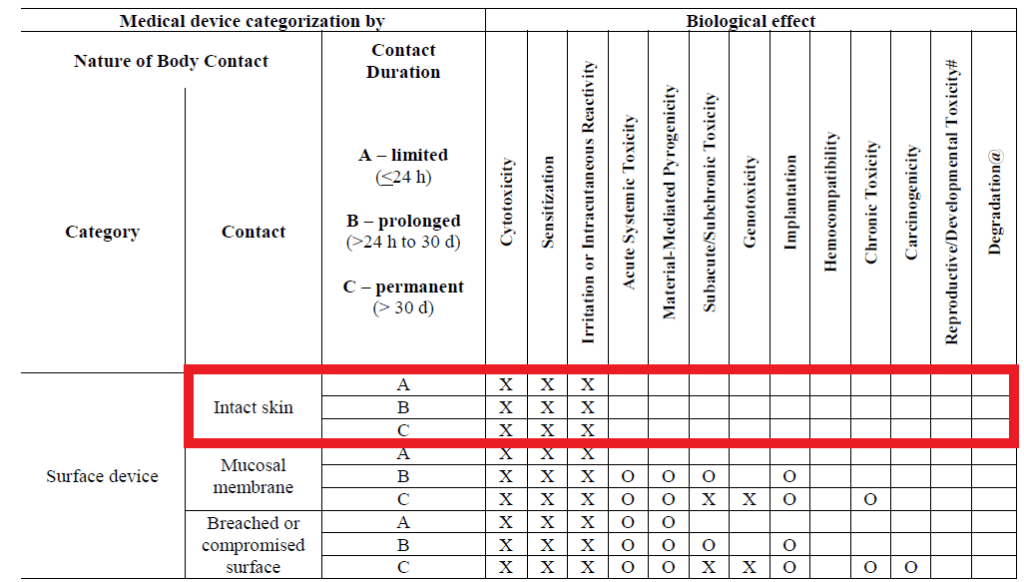
Fda Guidance Device Biocompatibility Intact Skin Namsa
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Looking Beyond Usp Class Vi Testing What Is Usp 87 Testing Holland Applied Technologies

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download
A Biocompatible Polycarbonate 3d Printing Material Stratasys

Printing The Strongest 3d Parts With Ultem 1010 Trimech
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

What Is Medical Grade Tpu Icp Das Biomedical Polymers
General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants